In the early stages of gene delivery, the method primarily involved directly transforming or transfecting plasmid DNA carrying the target gene (in this article, the term "gene" is not limited to protein-coding genes but also includes other nucleic acid sequences such as siRNA) into target cells. Although this method is simple to operate and low in cost, its transduction efficiency is relatively low, and gene expression stability is insufficient in some cell types. Additionally, plasmid DNA may have difficulty maintaining itself in cells over the long term and lacks efficient targeted delivery.
With the continuous development of gene function research and gene therapy, viral vectors have emerged as important tools in gene delivery due to their high transduction efficiency and strong targeting ability. These vectors can efficiently introduce genes into target cells to achieve gene expression, gene editing, gene repair, and other objectives. Common viral vectors include adenovirus (AdV), lentivirus (LV), and adeno-associated virus (AAV). Among these, adeno-associated virus (AAV) has garnered significant attention as a "star" vector in both research and gene therapy due to its low immunogenicity, long-term gene expression, and good tissue specificity, particularly in the treatment of genetic diseases.
What is AAV?
Adeno-associated virus (AAV) is a small, non-enveloped single-stranded DNA virus (approximately 4.8 kb) with an icosahedral structure about 20 nm in diameter. AAV is a simple structure single-stranded DNA defective virus that typically requires the help of helper viruses, such as adenoviruses or herpes simplex viruses, to induce pathogenic infection and generate progeny AAV.
AAV was first discovered in adenovirus preparations in 1965, from which it derives its name. With the rise of gene therapy technology in the 1990s, AAV became an ideal gene delivery tool due to its lack of significant clinical pathogenicity and its ability to achieve long-term stable gene expression in various cell types.
There are more than 100 mutant strains of AAV isolated from nature. Due to structural differences in the capsid proteins of different AAV serotypes, AAV shows varying abilities to bind to different cell surface receptors, making it capable of infecting specific organs, tissues, and cells.
In recent years, with the rapid development of gene editing technologies such as CRISPR-Cas9, the non-pathogenic wild-type AAV has been modified into recombinant adeno-associated virus vectors (rAAV). These vectors are widely used in gene expression, gene manipulation, and gene therapy and have become a core tool in many gene therapies that are either approved or in late-stage clinical development. rAAV is considered one of the most promising gene research and gene therapy vectors due to its variety, extremely low immunogenicity, high safety, ability to infect both dividing and non-dividing cells, strong diffusion ability, and long-lasting gene expression in vivo.
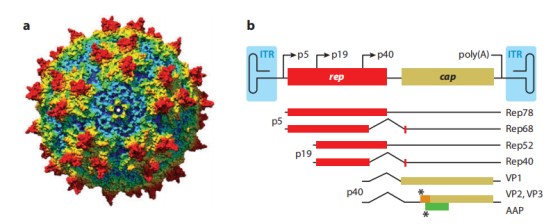
Figure 1. AAV Map [1]
a. Observation of the fivefold symmetry axis on the surface of the AAV bacteriophage (Chimera space-filling model)
b. AAV gene map
[1] Jude, R., et al. "AAV-Mediated Gene Therapy for Research and Therapeutic Purposes." Annual Review of Virology (2014).
Advantages of rAAV Vectors
1. High safety: AAV is a non-pathogenic virus. Wild-type AAV relies on the rep gene for low-frequency site-specific integration, while rAAV does not integrate into the host genome. The general consensus is that AAV viruses do not cause diseases in mammals or humans.
2. Low immunogenicity: rAAV induces a weak immune response, allowing it to function in the body over a long period of time.
3. Strong targeting ability: rAAV has multiple serotypes, each with specific affinity for different tissues and cells.
4. Good stability: rAAV vectors have good stability in vivo, with compact viral particles that are resistant to nucleases degradation.
5. Broad host cell range: rAAV can infect both dividing and non-dividing cells, allowing for long-term expression in cells with low proliferation.
6. Strong diffusion ability: rAAV vectors can effectively penetrate tissue barriers and achieve widespread gene delivery.
rAAV Packaging System
(1) rAAV Vector
Recombinant adeno-associated virus (rAAV) vectors are created by removing the rep and cap genes of natural AAV, retaining only the terminal repeat sequences (ITR) required for viral packaging. This effectively reduces immune responses and cytotoxicity. During construction, the exogenous gene is inserted between the two inverted ITR sequences in the viral genome plasmid (pAAV), ensuring that the gene can be expressed in the target cells. Since rAAV lacks the ability to self-package, packaging helper plasmids such as pCap/Rap are used to provide the required Rep and Cap proteins for the virus. The helper plasmid pAdHelper provides adenoviral genes (such as E2A, E4, VA) that promote efficient packaging and stable expression of rAAV.
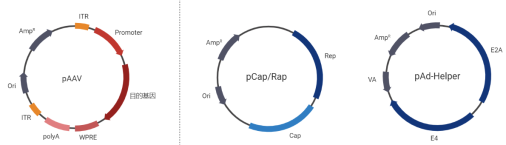
Figure 2. AAV Packaging Plasmids: Viral genome plasmid pAAV, packaging helper plasmids pCap/Rap and pAdHelper.
(2) Production Cells
Commonly used production cells, such as HEK293 cells, express the adenovirus E1 protein, providing the necessary auxiliary functions. By co-transfecting AAV packaging plasmids with helper plasmids into HEK293 cells, infectious rAAV particles are efficiently generated, completing the packaging process of the rAAV vector
rAAV Infection Mechanism
(1) Recognition and Binding: rAAV binds to receptors on the surface of the host cell via its capsid proteins, primarily including heparan sulfate proteoglycans (HSPGs) and certain specific cell surface receptors. Different serotypes of AAV capsids have unique structural sites that determine their specificity for recognizing certain glycosaminoglycan receptors and co-receptors.
(2) Endocytosis: After rAAV binds to cell surface receptors, it activates intracellular signaling pathways that trigger receptor-mediated endocytosis. rAAV particles are enveloped within endocytic vesicles and enter the cytoplasm of the cell.
(3) Nuclear Entry and Transport: The mechanism for rAAV genome nuclear entry remains inconclusive, but it is known that its genome enters the nucleus through nuclear pores. Once in the nucleus, the viral single-stranded DNA is repaired and converted into double-stranded DNA. Since AAV does not encode the enzymes required for replication, it relies on the host cell's replication machinery to stabilize its genome. During this process, the exogenous gene is transcribed into mRNA via the host cell's transcription machinery.
(4) Release and Expression: The single-stranded DNA of the rAAV genome is converted into double-stranded DNA, initiating the expression of the therapeutic gene. The mRNA produced through transcription is translated into the target protein in the cytoplasm, typically the protein encoded by the exogenous therapeutic gene. During this process, the structural genes of AAV (such as Rep and Cap) are not expressed, effectively reducing immune response and cytotoxicity.
(5) Sustained Expression and Immune Evasion: After rAAV infection, the viral genome sometimes exists in the nucleus in a circular form, allowing the exogenous gene to be expressed continuously over the long term without triggering significant immune responses. The low immunogenicity of rAAV makes it an ideal vector for gene therapy, suitable for scenarios requiring long-term or permanent treatment.
rAAV Regulatory Expression
To achieve precise control over gene expression, researchers have developed various rAAV-based strategies. Here are several common regulatory strategies:
|
Strategy
|
Construction
|
Function
|
Application
|
|
Target Gene Overexpression
|
Coding sequence (CDS) of the target gene
|
Achieves overexpression of the target gene
|
Gene function research, gene replacement therapy
|
|
Target Gene Expression Interference (RNAi)
|
RNA interference fragments of the target gene (e.g., shRNA or miRNA)
|
Reduces target gene expression via the RNAi mechanism
|
Gene function research, treatment of diseases caused by gene overexpression
|
|
Tissue-Specific or Inducible Expression
|
Tissue-specific promoter or inducible promoter (e.g., Tet-On system) + target gene
|
Regulates gene expression in specific tissues or under specific conditions
|
Research or therapy requiring tissue-specific or time-specific gene expression control
|
|
Gene Editing and Repair
|
CRISPR-Cas9 system AAV + Donor AAV co-transfection
|
Enables precise gene editing or repair
|
Mutation gene repair, precise gene editing
|
Selection of rAAV Serotypes
rAAV vectors have multiple serotypes, each with different affinities for various cells and tissues. When selecting a serotype, factors such as target tissue, delivery efficiency, and immune response should be considered. For example, AAV9 is commonly used for the central nervous system, while AAV2 is suitable for organs like the liver or heart. Tsingke offers a variety of rAAV serotypes for researchers to choose from and provides customized services based on specific needs.
✽ Multiple serotypes available
✽ Custom serotype options provided
✽ Multi-serotype trial packs available (containing various serotypes of AAV, each expressing a fluorescent reporter gene, suitable for systematic comparison of different serotypes and selecting the optimal serotype for specific applications).
Selection of Tissue-Specific Promoters for rAAV
To achieve precise gene expression, selecting an appropriate tissue-specific promoter is essential. The promoter in an rAAV vector determines the expression level and timing of the target gene, making it crucial to choose the right promoter based on research needs and experimental conditions. Commonly used promoters include CMV (a strong promoter for high expression), EF1α (for stable expression), and liver-specific promoters, among others.
Tsingke provides optimized promoter selection solutions based on customer expression requirements to ensure precise gene expression. Additionally, we offer rAAV serotypes targeting different organs and customized expression solutions by combining specific promoters.
Tsingke rAAV Product Catalog
|
Product Category
|
Product Name
|
Total Yield
|
|
Single-Stranded AAV (ssAAV)
|
High-Purity ssAAV Custom Packaging (Regular Yield Serotypes) – Includes Overexpression, RNA Interference (shRNA), and Knockout (CRISPR-SaCas9)
|
5E+11 VGS, 1E+12 VGS, 2E+12 VGS, 5E+12 VGS, 1E+13 VGS, 2E+13 VGS, 5E+13 VGS, 1E+14 VGS
|
|
High-Purity ssAAV Custom Packaging (Ultra-Low Yield Serotypes) – Includes Overexpression, RNA Interference (shRNA), and Knockout (CRISPR-SaCas9)
|
1E+12 VGS, 2E+12 VGS, 5E+12 VGS, 1E+13 VGS, 2E+13 VGS, 5E+13 VGS, 1E+14 VGS
|
|
AAV Interference (shRNA)/Knockout (CRISPR-SaCas9) – Three Targets (Regular Yield)
|
5E+11 VGS, 1E+12 VGS, 5E+12 VGS, 1E+13 VGS, 2E+13 VGS, 5E+13 VGS
|
|
AAV Interference (shRNA)/Knockout (CRISPR-SaCas9) – Three Targets (Ultra-Low Yield)
|
5E+11 VGS, 1E+12 VGS, 5E+12 VGS, 1E+13 VGS, 2E+13 VGS, 5E+13 VGS
|
|
Self-Complementary AAV (scAAV)
|
High-Purity scAAV Custom Packaging
|
5E+11 VGS, 1E+12 VGS, 2E+12 VGS, 5E+12 VGS, 1E+13 VGS, 2E+13 VGS, 5E+13 VGS, 1E+14 VGS, 2E+14 VGS
|
As a core vector for gene therapy, AAV is driving rapid advancements in the field due to its high efficiency, safety, and precision. With ongoing technological progress, AAV holds great promise for treating genetic disorders, cancer, and neurodegenerative diseases, and is expected to become a crucial tool in precision medicine.
Tsingke is dedicated to optimizing AAV vector technology, providing cutting-edge gene delivery solutions to support the development of gene therapy and contribute to the advancement of healthcare.