


Our cell line construction services provide precise and reliable genetic modifications to support diverse research needs. We offer monoclonal and pooled cell lines for knockout, knock-in, overexpression, knockdown, and point mutation studies, with validation at both genomic and protein levels. Each cell line is rigorously characterized to ensure accurate gene editing, stable expression, and reproducible experimental outcomes. With professional project management, rapid turnaround, and comprehensive reporting, our services save valuable time and reduce experimental uncertainty, empowering accelerated progress in functional genomics, drug discovery, and molecular biology research.
| Stable Cell Line | Deliverables | Turnaround Time |
| Monoclonal KO Cell | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. | From 8 weeks |
| KO cell pool | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. | From 6 weeks |
| Monoclonal Overexpression/Knockdown Cell | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. Control cell lines, 2 vials, 1E+6 cells/clone. | From 8 weeks |
| Overexpression/Knockdown Cell pool | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. Control cell lines, 2 vials, 1E+6 cells/clone. | From 6 weeks |
| Homozygous Monoclonal Point Mutation Cell Line | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. Control cell lines, 2 vials, 1E+6 cells/clone. | From 11 weeks |
| Monoclonal Knock-in Cell | Cryopreserved or flask-cultured positive clone cell lines, 1 vial, ≥1E+6 cells/clone. Control cell lines, 2 vials, 1E+6 cells/clone. | From 11 weeks |
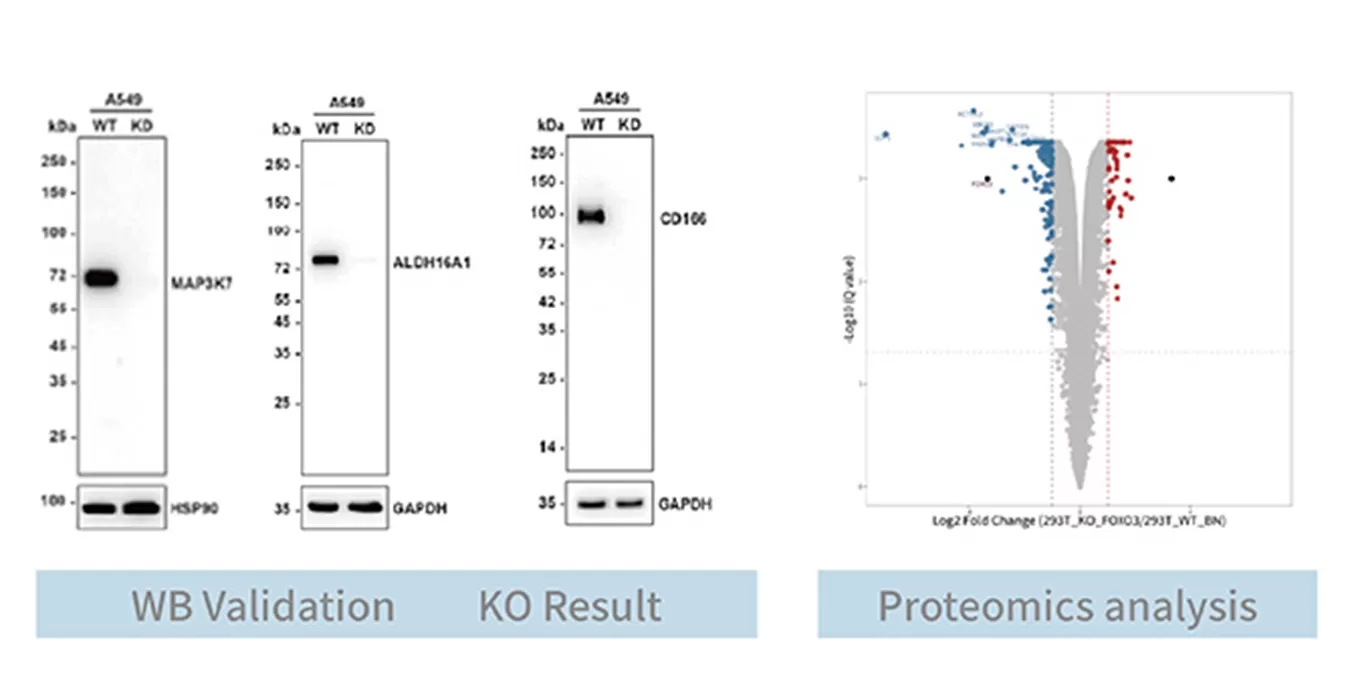
| Note: Western blotting or proteomics validation can be performed for monoclonal cell lines. | ||

Knockout Cell Line Construction: Verified by western blot (WB) or proteomics to to confirm knockout at the protein level.
Polyclonal stable cell line (cell pool): This is obtained by directly selecting cells with drug resistance after transfection. It consists of multiple clones from different sources, with varying integration sites and expression levels of the exogenous gene. It has a short production cycle and low cost, suitable for initial screening and high-expression applications.
Monoclonal stable cell line: This is derived from a cell pool through methods such as limiting dilution, and consists of a single clone of cells with stable integration of the exogenous fragment. All cells have the same integration site, ensuring consistency and stability in expression. It is suitable for high-precision research and biopharmaceutical production. However, it has a longer production cycle and higher cost, and is ideal for cell line experiments requiring subcellular localization, difficult-to-infect cells, and low-expression applications.