



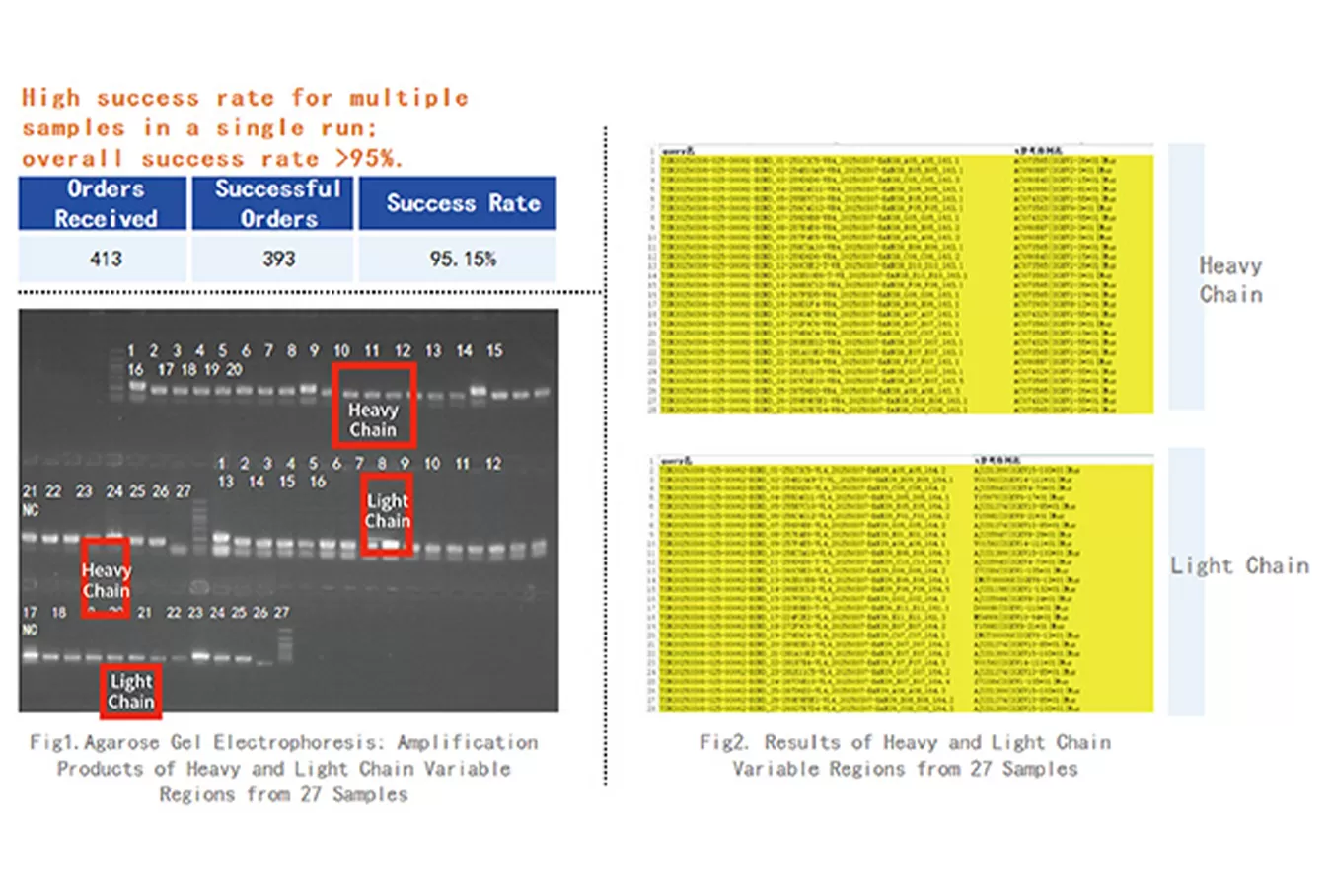
Our hybridoma sequencing service delivers fast, precise identification and validation of monoclonal antibody sequences from hybridoma cell lines. We offer three tailored sequencing options: Variable Region Sequencing for accurate determination of light and heavy chain variable regions, Full-Length Sequencing for comprehensive antibody sequences, and NGS High-Throughput Sequencing for rapid, large-scale validation of PCR-amplified products. Designed to overcome the limitations of traditional hybridoma analysis, our service ensures quick clone authentication, reliable recombinant expression, and seamless downstream applications, accelerating antibody characterization, optimization, and therapeutic research with confidence, speed, and high-quality results.
| Service | Turnaround Time | Details | Deliverables |
| Variable Region Sequencing | 1–2 weeks | Identification of antibody light and heavy chain variable regions with high accuracy. | Electronic Report including: 1) Light and heavy chain sequences 2) Raw sequencing data (NGS without chromatogram files) |
| Full-Length Sequencing | 1–2 weeks | Amplification and sequencing of complete antibody sequences. | |
| NGS High-Throughput Sequencing | 1–2 weeks | Next-generation sequencing validation of PCR products for reliable results. |

Customers should provide more than 1 × 10⁶ frozen hybridoma cells or Trizol-processed cells. Antibody subtype information and hybridoma species/source details are also needed.